Preeclampsia/Eclampsia
Model Inputs \(\rightarrow\) Obstetric Complications \(\rightarrow\) Preeclampsia/Eclampsia
Overview
Hypertensive disorders of pregnancy refer to a range of conditions associated with high blood pressure, proteinuria and, rarely, seizures. Severe preeclampsia and eclampsia have the highest case fatality rates of the hypertensive disorders of pregnancy, and can lead to placental abruption, disseminated intravascular coagulopathy (DIC), adult respiratory distress syndrome (ARDS), cerebral hemorrhage, seizures, and death.[1] While medication can alleviate the symptoms and their negative effects, the only cure for the pregnant woman is expedited delivery.[2]
The etiology of the condition remains unclear. It is thought to arise from the placenta and is associated with malfunction of the lining of blood vessels. The clinical spectrum of disease in preeclampsia varies, ranging from mild, asymptomatic disease, often occurring close to term, to severe, uncontrolled hypertension typically developing remote from term (less than 34 weeks).[3] Eclampsia and preeclampsia tend to occur more frequently in the second half of pregnancy, and can also occur postpartum, with up to 26% of eclamptic seizures occurring beyond 48 hours and as late as six weeks following delivery.[4,5] Preeclampsia and eclampsia are associated with perinatal deaths, abruption placenta, and cardiovascular disease later in life.[2]
The initial assessment of a woman presenting with symptoms suggestive of preeclampsia/eclampsia includes assessment of blood pressure, testing of urine for proteins, observation for the presence of convulsions, and taking the fetal heart rate. A woman with severe preeclampsia presents with high blood pressure (>160/110 mm Hg) and proteinurea; in addition, eclampsia is associated with convulsions, and needs to be managed with oxygen, intravenous fluids, anti-hypertensives and anticonvulsants. Emergency transportation has to be arranged to a facility that can offer follow-up care after initial management of preeclampsia/eclampsia, and which has facilities for induction of labor or operative delivery if needed. During follow up care, blood pressure and fluid intake have to be closely monitored, the patient needs to be monitored for convulsions and symptoms related to magnesium toxicity, and the option of inducing labor or operative delivery should be considered.[6]
Data
Incidence
Preeclampsia/eclampsia (PE/E) is a relatively frequent pregnancy complication, with reported incidence estimates of 2%-8%.[1] However, geographic, social, economic, and racial differences are thought to be responsible for incidence rates up to 3 times higher in some populations.[7] Generalized seizures (eclampsia) occur in up to 8% of women with preeclampsia in developing countries, a rate 10-30 times higher than in developed countries.[8]
A systematic review covering 39 million women in 40 countries estimated an incidence of 4.6% (95% CI 2.7-8.2) for preeclampsia among all deliveries, and 1.4% (95% CI 1.0-2.0) for eclampsia,[9] while a secondary analysis of WHO data found the combined prevalence of PE/E to be 4%.[10] There was high regional variation, with sub-Saharan Africa estimated to have the highest prevalence.[9] A separate secondary analysis of the WHO Multicountry Survey on Maternal and Newborn Health (WHOMCS) found incidences of 2.16% for pre-eclampsia, 0.28% for eclampsia, and 0.29% for chronic hypertension.[11] This is similar to previous figures by the WHO which estimated that preeclampsia occurred in 3.2% of live births, and eclampsia occurred in 0.5%.[12] A WHO multi-country survey on maternal and newborn health estimated that preeclampsia is associated with over 25 percent of severe maternal outcomes and is the direct cause of 20 percent of reported maternal deaths.[13]
A analysis of DHS data on 55,384 live deliveries from six surveys during 2005-2012 in Colombia, Bangladesh, Indonesia, Mali, Niger, and Peru found that indications of eclampsia were recorded for 1.2% (95% CI 1.0–1.3), 1.7% (95% CI 1.5–2.1), and 1.7% (95% CI 1.5–2.1) of deliveries reported from the American, South East Asian, and African regions, respectively.[14] A prospective cohort study in the US estimated a prevalence of preeclampsia of 9.0%,[15] while a nationally representative study in the US using data from the National Hospital Discharge Survey from 1988 to 1997 of approximately 300,000 deliveries estimated that eclampsia was reported at 1.0 per 1,000 deliveries (95% CI 0.9-1.2), and that the overall incidence of hypertensive disorders in pregnancy was 5.9% (95% CI 5.2-6.5).[16] A cohort study of all women giving birth in 1998-2000 in Scandinavia estimated that the incidence of eclampsia was 5.0/10,000 deliveries (95% CI 4.3-5.7), with 86% being diagnosed with preeclampsia before the seizure, and 90% of seizures occurring after admission to hospital.[17] A national surveillance study in the UK in 1992 recorded an incidence of 4.9/10,000 deliveries, while an updated population-based study using the UK Obstetric Surveillance System (UKOSS) for all women delivering in the UK in 2005-2006 found the incidence of eclampsia was 2.7 cases per 10,000 births (95% CI 2.4–3.1).[18]
Several risk factors for PE/E have been identified, including increasing maternal age (age 30-34 AOR: 1.40 [95% CI 1.31-1.51], age 35+ AOR: 1.95 [95% CI 1.80-2.12]), severe anemia (AOR: 2.38 [95% CI 1.86-3.05]), and multiple gestation (AOR: 2.96 [95% CI 1.74-5.03]).[10,15] Other risk factors include high body mass index, chronic hypertension, and gestational diabetes. In clinical practice, although these factors predict just 30% of women who develop PE/E,[19] women with a history of PE/E in a previous pregnancy have an increased risk (AOR: 3.63 [95% CI 2.29-5.73]) of developing it again.[15]
Morbidity/Mortality
Eclampsia has a high case fatality rate, which varies among regions of the world, presumably as a function of the access to and quality of health care.[20] Although eclampsia is associated with an increased risk of maternal death in developed countries (0%-1.8%), the mortality rate is as high as 15% in developing countries.[7] A retrospective study of PE/E-related deaths found that access to and delay in seeking care was a major determinant of mortality, with 37.7% in grade IV coma and 54% with recurrent convulsions prior to admission.[21] Concurrent eclampsia with HELLP Syndrome was found to be linked in 5-6 out of every 10 deaths associated with either condition.[22]
Graham 2006[23] reports an overall case fatality rate of 1.7% for PE/E, while a study in India of 864 eclampsia patients reported a case fatality rate of 7.29%.[24] These women were reported to all have been treated with magnesium sulphate, and nearly half had caesarean sections, implying that this CFR may be low compared to ‘natural history’ outcomes. An earlier study showed that severe PE/E required treatment with intravenous hydralazine and magnesium sulphate and in addition, approximately 10% of all cases were assumed to require emergency cesarean section.[25] A study of eclampsia in University of Benin Teaching Hospital, Nigeria during 1995-2002 found 11 maternal deaths occurred among 103 cases of eclampsia, with a case fatality rate of 10.7%.[26] A similar study of eclampsia in Dar es Salaam, Tanzania found a case fatality rate of 5.0% for women who delivered at Muhimibili National Hospital, and 16% for those referred to the hospital after delivering elsewhere.[27]
Hypertensive disorders of pregnancy are also associated with increased maternal morbidity, predominantly because of maternal stroke.[28,29] While the general incidence of stroke during pregnancy ranges from 10 to 34 per 100,000 deliveries,[30] women with a history of preeclampsia have approximately double the risk for fatal and nonfatal stroke.[31,32]
In addition to maternal morbidity/mortality, PE/E increases the risk of stillbirth and neonatal mortality. A population-based study in the UK in 2005-2006 found that 8/222 infants delivered by mothers with eclampsia were stillborn and 5 died in the neonatal period.[18] PE/E may contribute to poor fetal growth and have an estimated population attributable risk for stillbirths of about 5%.[33] The risk of fetal demise for women with preeclampsia has been estimated at 5.2 per 1,000 pregnancies,[34] and an analysis of DHS data found that eclampsia was associated with a two-fold increase (95% CI 1.4–3.2) in the risk of early neonatal mortality.[14]
Parameters
We used a hierarchical logistic regression model to model incidence of PE/E, based on priors estimated on data from Abalos 2013[9] and Bilano 2014[10]. In addition, we account for the following individual-level risk factors: maternal age (30-34, 35+), severe anemia, multiple gestation, and history of PE/E. We enforce non-decreasing RRs by increasing maternal age when sampling. Conditional on developing PE/E we model risks of mortality, neurological sequelae, and stillbirth, with higher risks for eclampsia vs preeclampsia.
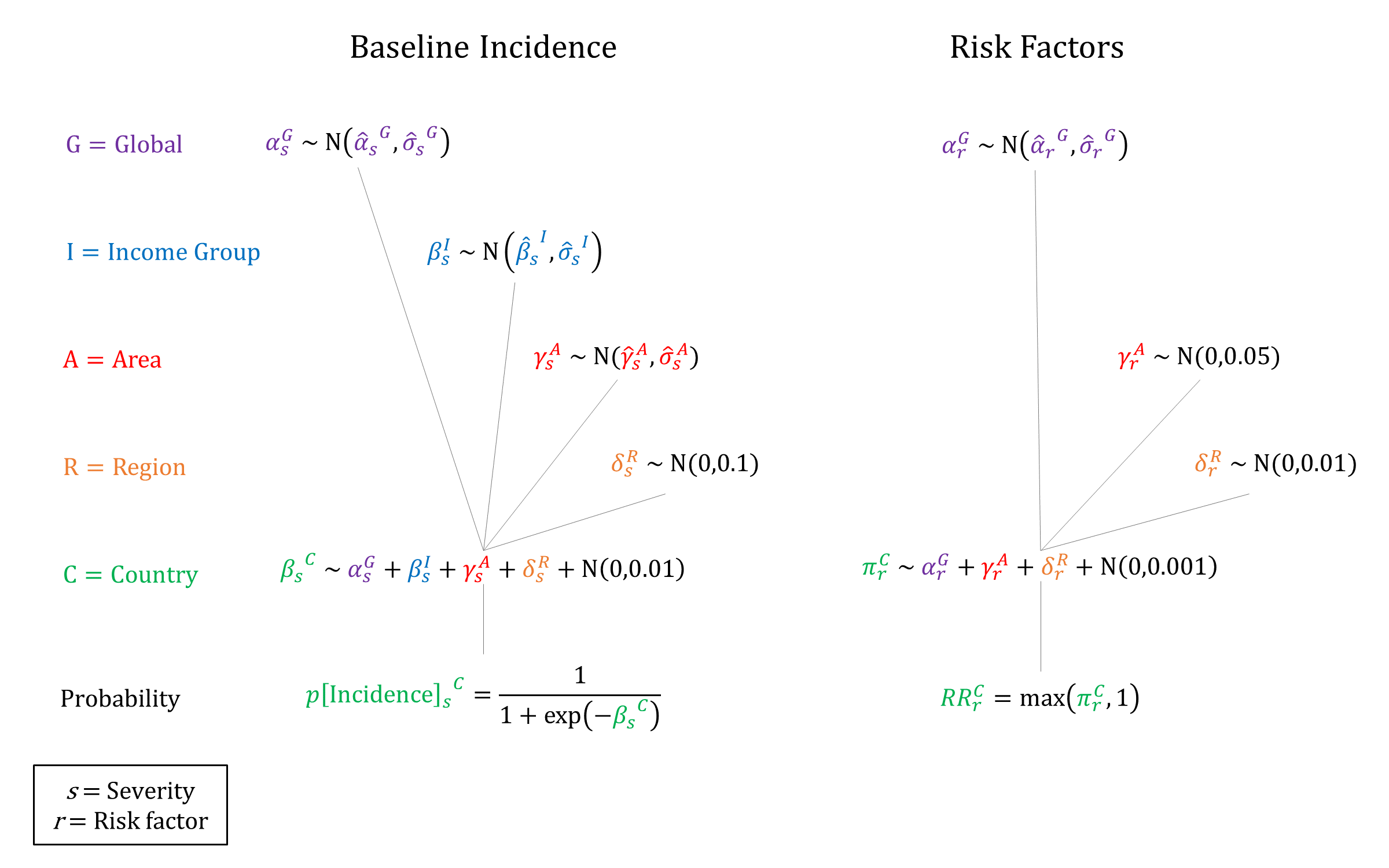
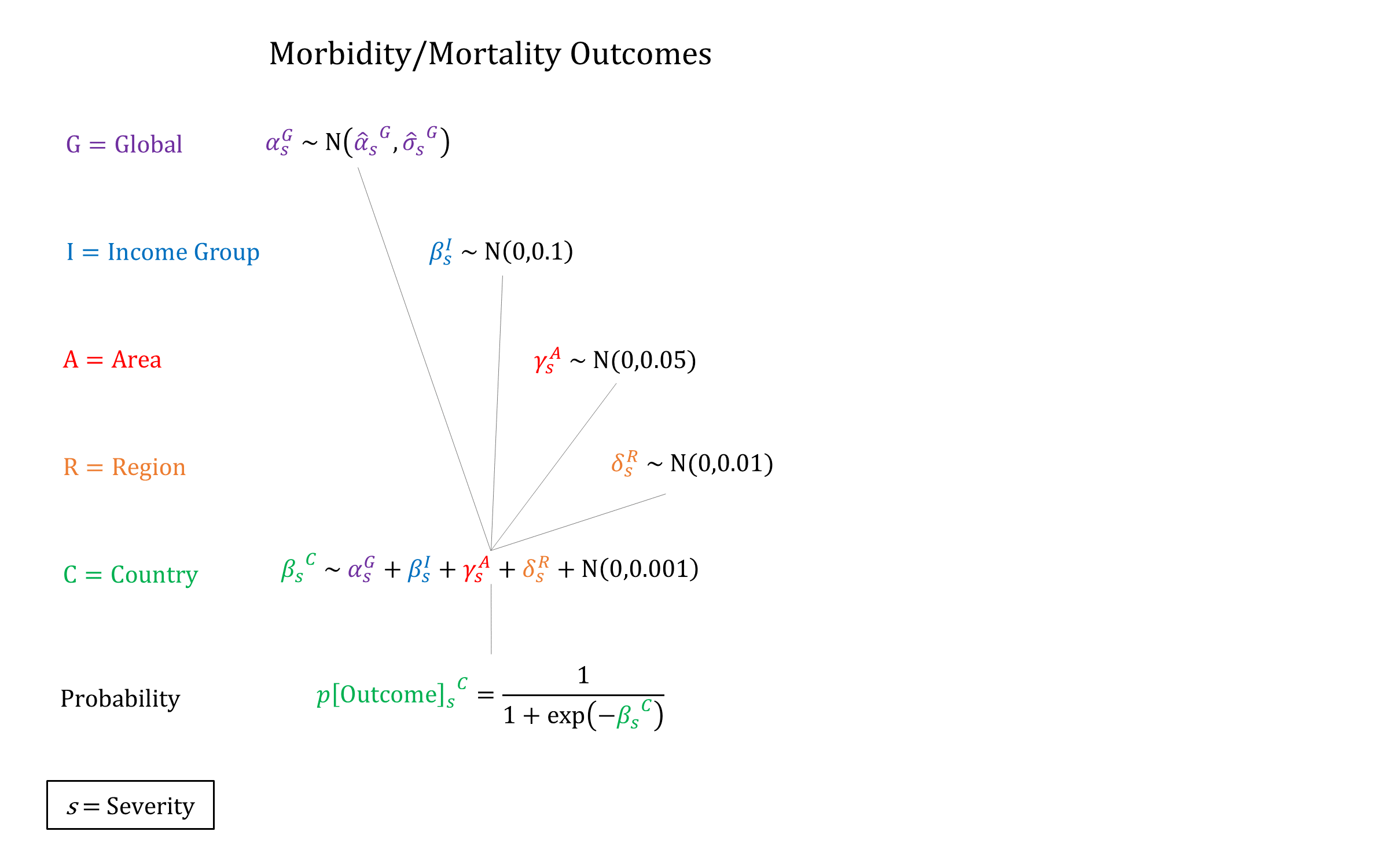
Priors
Model Implementation
At the time of delivery, the incidence and outcomes of PE/E are simulated, together with the availability and efficacy of interventions that modify the ‘natural history’ probabilities. We assume that the risk factors that affect incidence are independent in the model. While we model the risk of stillbirths for women who develop PE/E, we do not currently take into account neonatal outcomes in the model.
References
- Duley L. The global impact of pre-eclampsia and eclampsia. Semin Perinatol 2009; 33(3): 130-7. DOI: https://doi.org/10.1053/j.semperi.2009.02.010
- Filippi V, Chou D, Ronsmans C, Graham W, Say L. Chapter 3. Levels and causes of maternal mortality and morbidity. In: Black RE, Laxminarayan L, Walker N, Temmerman M, eds. Disease control priorities 3rd edn. Volume 2. Reproductive, maternal, newborn and child health. Washington, DC: World Bank, 2016. Available at: https://www.ncbi.nlm.nih.gov/books/NBK361917/
- Gülmezoglu AM, Lawrie TA, Hezelgrave N, et al. Chapter 7. Interventions to Reduce Maternal and Child Morbidity and Mortality. In: Black RE, Laxminarayan L, Walker N, Temmerman M, eds. Disease control priorities 3rd edn. Volume 2. Reproductive, maternal, newborn and child health. Washington, DC: World Bank, 2016. Available at: https://www.ncbi.nlm.nih.gov/books/NBK361904/
- Al-Safi Z, Imudia AN, Filetti LC, Hobson DT, Bahado-Singh RO, Awonuga AO. Delayed postpartum preeclampsia and eclampsia: demographics, clinical course, and complications. Obstet Gynecol 2011; 118(5): 1102-7. DOI: https://doi.org/10.1097/aog.0b013e318231934c
- Rawlins B, Plotkin M, Rakotovao JP, et al. Screening and management of pre-eclampsia and eclampsia in antenatal and labor and delivery services: findings from cross-sectional observation studies in six sub-Saharan African countries. BMC Pregnancy Childbirth 2018; 18(1): 346. DOI: https://doi.org/10.1186/s12884-018-1972-1
- Jayanna K, Mony P, Ramesh BM, et al. Assessment of facility readiness and provider preparedness for dealing with postpartum haemorrhage and pre-eclampsia/eclampsia in public and private health facilities of northern Karnataka, India: a cross-sectional study. BMC Pregnancy Childbirth 2014; 14: 304. DOI: https://doi.org/10.1186/1471-2393-14-304
- Ghulmiyyah L, Sibai B. Maternal mortality from preeclampsia/eclampsia. Semin Perinatol 2012; 36(1): 56-9. DOI: https://doi.org/10.1053/j.semperi.2011.09.011
- Steegers EA, von Dadelszen P, Duvekot JJ, Pijnenborg R. Pre-eclampsia. Lancet 2010; 376(9741): 631-44. DOI: https://doi.org/10.1016/s0140-6736(10)60279-6
- Abalos E, Cristina C, Grosso AL, Chou D, Say L. Global and regional estimates of preeclampsia and eclampsia: a systematic review. European Journal of Obstetrics and Gynecology and Reproductive Biology 2013; 170(1): 1-7. DOI: https://doi.org/10.1016/j.ejogrb.2013.05.005
- Bilano VL, Ota E, Ganchimeg T, Mori R, Souza JP. Risk factors of pre-eclampsia/eclampsia and its adverse outcomes in low- and middle-income countries: a WHO secondary analysis. PLoS ONE 2014; 9(3): e91198. DOI: https://doi.org/10.1371/journal.pone.0091198
- Abalos E, Cuesta C, Carroli G, et al. Pre-eclampsia, eclampsia and adverse maternal and perinatal outcomes: a secondary analysis of the World Health Organization Multicountry Survey on Maternal and Newborn Health. BJOG 2014; 121(S1): 14-24. DOI: https://doi.org/10.1111/1471-0528.12629
- AbouZahr C, Wardlaw T. Maternal mortality in 2000: estimates developed by WHO, UNICEF and UNFPA. Department of Reproductive Health and Research, World Health Organization: Geneva, 2004. Available at: https://apps.who.int/iris/handle/10665/42930
- Souza JP, Gülmezoglu AM, Vogel J, et al. Moving beyond essential interventions for reduction of maternal mortality (the WHO Multicountry Survey on Maternal and Newborn Health): a cross-sectional study. Lancet 2013; 381(9879): 1747-55. DOI: https://doi.org/10.1016/s0140-6736(13)60686-8
- Bellizzi S, Sobel HL, Ali MM. Signs of eclampsia during singleton deliveries and early neonatal mortality in low- and middle-income countries from three WHO regions. Int J Gynaecol Obstet 2017; 139(1): 50-54. DOI: https://doi.org/10.1002/ijgo.12262
- Paré E, Parry S, McElrath TF, Pucci D, Netwon A, Lim KH. Clinical risk factors for preeclampsia in the 21st century. Obstet Gynecol 2014; 124(4): 763-70. DOI: https://doi.org/10.1097/aog.0000000000000451
- Zhang J, Meikle S, Trumble A. Severe maternal morbidity associated with hypertensive disorders in pregnancy in the United States. Hypertens Pregnancy 2003; 22(2): 203-12. DOI: https://doi.org/10.1081/prg-120021066
- Andersgaard AB, Herbst A, Johansen M, et al. Eclampsia in Scandinavia: Incidence, substandard care, and potentially preventable cases. Acta Obstet Gynecol Scand 2006; 85(8): 929–936. DOI: https://doi.org/10.1080/00016340600607149
- Knight M, UKOSS. Eclampsia in the United Kingdom 2005. BJOG 2007; 114(9): 1072-8. DOI: https://doi.org/10.1111/j.1471-0528.2007.01423.x
- Mol BWJ, Roberts CT, Thangaratinam S, Magee LA, de Groot CJM, Hofmeyr GJ. Pre-eclampsia. Lancet 2016; 387(10022): 999-1011. DOI: https://doi.org/10.1016/s0140-6736(15)00070-7
- Dolea C, AbouZahr C. Global Burden of Hypertensive Disorders in Pregnancy in the Year 2000. Evidence and information for policy (EIP). Global Burden of Disease Working Paper, 2003. Available at: https://www.who.int/healthinfo/statistics/bod_hypertensivedisordersofpregnancy.pdf
- Sawhney H, Aggarwal N, Biswas R, Vasishta K, Gopalan S. Maternal mortality associated with eclampsia and severe preeclampsia of pregnancy. J Obstet Gynaecol Res 2000; 26(5): 351-6. DOI: https://doi.org/10.1111/j.1447-0756.2000.tb01338.x
- Vigil-De Gracia P. Maternal deaths due to eclampsia and HELLP syndrome. Int J Gynaecol Obstet 2009; 104(2): 90-94. DOI: https://doi.org/10.1016/j.ijgo.2008.09.014
- Graham WJ, Cairns J, Bhattacharya S, Bullough CHW, et al. Maternal and Perinatal Conditions. In: Jamison DT, Breman JG, Measham AR, et al, eds. Disease Control Priorities in Developing Countries. 2nd edition. Washington (DC): World Bank; 2006. Available at: https://www.ncbi.nlm.nih.gov/books/NBK11742/
- Biswas A, Modak R, Baksi S, Biswas S. Epidemiological study of eclampsia in a referral teaching hospital. J Indian Med Associ 2005; 103(6): 323-4, 326. PMID: https://pubmed.ncbi.nlm.nih.gov/16225158/
- Cahuana-Hurtado L, Sosa-Rubi S, Bertozzi S. Cost of mother-child care in Morelos State. Salud Publica Mex 2004; 46(4): 316-25. DOI: https://doi.org/10.1590/s0036-36342004000400006
- Onuh SO, Aisien AO. Maternal and fetal outcome in eclamptic patients in Benin City, Nigeria. J Obstet Gynaecol 2004; 24(7): 765-8. DOI: https://doi.org/10.1080/01443610400009451
- Urassa DP, Carlstedt A, Nyström L, Massawe SN, Lindmark G. Eclampsia in Dar es Salaam, Tanzania – Incidence, outcome, and the role of antenatal care. Acta Obstet Gynecol Scand 2006; 85(5): 571-8. DOI: https://doi.org/10.1080/00016340600604880
- Martin JN Jr, Thigpen BD, Moore RC, Rose CH, Cushman J, May W. Stroke and severe preeclampsia and eclampsia: a paradigm shift focusing on systolic blood pressure. Obstet Gynecol 2005; 105(2): 246-254. DOI: https://doi.org/10.1097/01.aog.0000151116.84113.56
- Folk DM. Hypertensive Disorders of Pregnancy: Overview and Current Recommendations. J Midwifery Womens Health 2018; 63(3): 289-300. DOI: https://doi.org/10.1111/jmwh.12725
- Bushnell C, Chireau M. Preeclampsia and Stroke: Risks during and after Pregnancy. Stroke Res Treat 2011; 2011: 858134. DOI: https://dx.doi.org/10.4061%2F2011%2F858134
- Bellamy L, Casas JP, Hingorani AD, Williams DJ. Pre-eclampsia and risk of cardiovascular disease and cancer in later life: systematic review and meta-analysis. BMJ 2007; 335(7627): 974. DOI: https://doi.org/10.1136/bmj.39335.385301.be
- McDonald SD, Malinowski A, Zhou Q, Yusuf S, Devereaux PJ. Cardiovascular sequelae of preeclampsia/eclampsia: a systematic review and meta-analyses. Am Heart J 2008; 156(5): 918-930. DOI: https://doi.org/10.1016/j.ahj.2008.06.042
- Lawn JE, Blencowe H, Wasiwa P, et al. Stillbirths: rates, risk factors, and acceleration towards 2030. Lancet 2016; 387(10018): 587-603. DOI: https://doi.org/10.1016/S0140-6736(15)00837-5
- Harmon QE, Huang L, Umbach DM, et al. Risk of fetal death with preeclampsia. Obstet Gynecol 2015; 125(3): 628-635. DOI: https://doi.org/10.1097/aog.0000000000000696
GMatH (Global Maternal Health) Model - Last updated: 28 November 2022
© Copyright 2020-2022 Zachary J. Ward
zward@hsph.harvard.edu