Ectopic Pregnancy
Model Inputs \(\rightarrow\) Biological Parameters \(\rightarrow\) Ectopic Pregnancy
Overview
Ectopic pregnancy is a pregnancy in which the fertilized ovum implants outside the intrauterine cavity, with more than 95% occurring in the fallopian tubes.[1] Because these sites can not accommodate placental attachment or embryo growth, the potential for rupture and hemorrhage make ectopic pregnancy a high risk condition and the leading cause of maternal mortality in the first trimester.[1,2]
Prompt ultrasound evaluation is key in diagnosing ectopic pregnancy.[2] Appropriate treatment for patients with nonruptured ectopic pregnancy may include expectant management, medical management with methotrexate, or surgery. Surgical treatment is appropriate if ruptured ectopic pregnancy is suspected.[2]
Data
Incidence
Ectopic pregnancies occur in around 2% of pregnancies, with increasing risk for older women.[3,4,5] Andersen (2000)[6] presents rates of ectopic pregnancy by maternal age group from a population-based register linkage study in Denmark. After adjusting the denominator by subtracting all induced abortions from the number of pregnancies, the adjusted risk of ectopic pregnancy was calculated by age group. A population-based study in France found similar effect sizes for increased risk of ectopic pregnancy by maternal age.[7]
Prior ectopic pregnancy is a strong risk factor for recurrent ectopic pregnancy, with recurrence rates reported from 10% to 25%,[8,9] or 10 times the risk of the general population. For example, Bhattacharya 2012[10] reports an adjusted hazard rate of 13.0 (95% CI 11.63-16.86) and Jacob 2017[4] reports an adjusted odds ratio of 8.17 (95% CI 4.87-13.69).
Although many women diagnosed with ectopic pregnancy have no identifiable risk factors, a number of other risk factors have been associated with ectopic pregnancy, including smoking, alcohol, tubal surgery or tubal damage from sexually transmitted infections (STI), prior pelvic infection, IUD use, and pregnancy conceived by assisted reproduction.[4,8,11,12,13,14,15] Some of these risk factors may be associated with maternal age. For example, increased risk of ectopic pregnancies in teenage women is most likely caused by pelvic inflammatory disease.[6]
We model risk by maternal age for first-time ectopic pregnancy. For women with a previous ectopic pregnancy we model a risk of recurrence. Using a hierarchical model with country-specific effects allows us to account for different underlying risks not explicitly modeled, such as STI prevalence and assisted reproduction.
Morbidity/Mortality
Tubal pregnancies may either diminish in size and spontaneously resolve, or continue to grow and eventually lead to tubal rupture. However, there are no reliable clinical, sonographic, or biological markers (e.g. serum beta hCH or serum progesterone) that can predict rupture of a tubal ectopic pregnancy.[16] In addition, the time from conception to tubal rupture is short, often occurring within 6-8 weeks of conception.[13]
Reported tubal rupture rates range from population-based reports of 18%,[17] to clinic/physician office-based reports of 32%,[18] to hospital-based reports of 80% and higher.[19] For example, an analysis of 80 cases of ectopic pregnancy in Pakistan found a rupture rate over 90%,[20] likely due to selection bias of ruptured cases presenting at hospital.
In contrast, a study of women on Medicaid in the US found that ectopic pregnancy-associated complications occurred in only 11% of cases, with a mortality ratio of 0.48 per 100,000 live births.[21] Another study found that the ectopic pregnancy mortality ratio in the US declined from 1.15 to 0.50 deaths per 100,000 live births between 1980-1984 and 2003-2007.[22] In a review of African developing countries, a majority of hospital-based studies reported ectopic pregnancy case fatality rates (CFR) of 1-3% (10 times higher than reported in developed countries), with late diagnosis and emergency surgical treatment likely accounting for the high fatality rates.[23]
Because CFR estimates are based on diagnosed cases, we condition CFR on rupture in the model. We assume that the probability of rupture is around 10-20%, based on the population-based studies.[17,21] We assume that given rupture, the CFR is around 3-5% in the absence of treatment (i.e. natural history). Although tubal rupture seriously affects the current health of the mother, it seems to have no effect on subsequent fertility.[17] We therefore assume that women who survive a ruptured ectopic pregnancy have no continuing morbidity, besides the increased risk of recurrent ectopic pregnancy in the future.
Parameters
Incidence
We fit quadratic B-splines to the midpoints of the age groups, based on data from Andersen 2000.[6] Spline Width: 40
| Spline # | Knot Location | Height |
|---|---|---|
| 1 | 8 | \(N(0.03,0.002)\) |
| 2 | 22 \(^1/_3\) | \(N(0.01,0.002)\) |
| 3 | 37 \(^1/_3\) | \(N(0.05,0.002)\) |
| 4 | 52 | \(N(0.11,0.015)\) |
We extended the probability from age 50 to older ages.
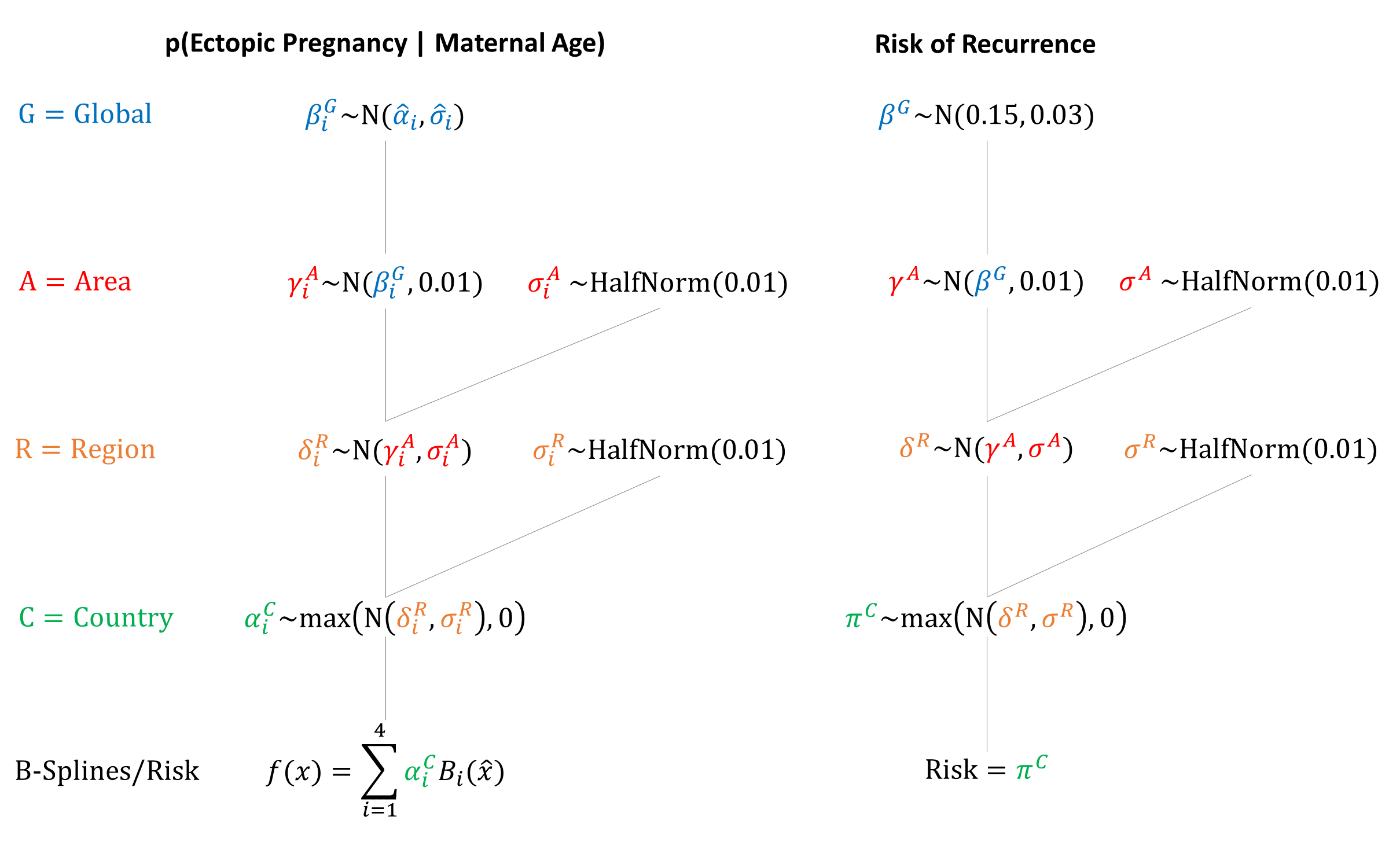
Morbidity/Mortality
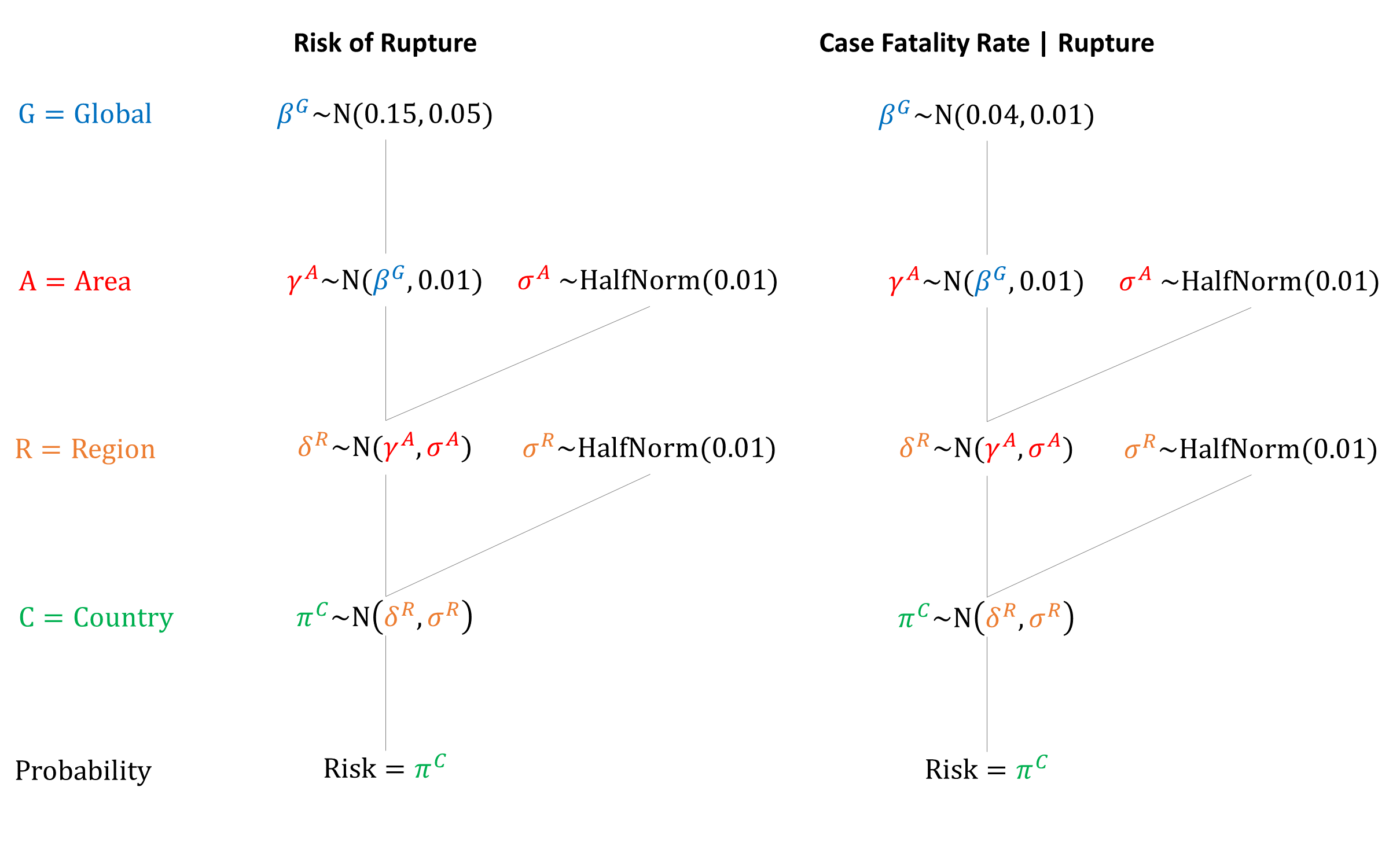
Priors
Model Implementation
Following conception in the model, the risk of ectopic pregnancy is simulated based on a woman’s age and her history of previous ectopic pregnancy. We assume that ectopic pregnancies happen during the first trimester (i.e. month 3) in the model.
References
- Tenore JL. Ectopic pregnancy. Am Fam Physician 2000; 61(4): 1080-8. https://www.aafp.org/afp/2000/0215/p1080.html
- Lozeau AM, Potter B. Diagnosis and management of ectopic pregnancy. Am Fam Physician 2005; 72(9): 1707-14. https://www.aafp.org/afp/2005/1101/p1707.html
- Stulberg DB, Cain LR, Dahlquist I, Lauderdale DS. Ectopic pregnancy rates in the Medicaid population. Am J Obstet Gynecol 2013; 208(4): 274.e1-7. DOI: https://doi.org/10.1016/j.ajog.2012.12.038
- Jacob L, Kalder M, Kostev K. Risk factors for ectopic pregnancy in Germany: a retrospective study of 100,197 patients. Ger Med Sci 2017; 15: Doc19. DOI: https://doi.org/10.3205/000260
- Ranji GG, Usha Rani G, Varshini S. Ectopic Pregnancy: Risk Factors, Clinical Presentation and Management. J Obstet Gynaecol India 2018; 68(6): 487-492. DOI: https://doi.org/10.1007/s13224-017-1075-3
- Andersen AM, Wohlfahrt J, Christens P, Olsen J, Melbye M. Maternal age and fetal loss: population based register linkage study. BMJ 2000; 320(7251): 1708-12. DOI: https://doi.org/10.1136/bmj.320.7251.1708
- Bouyer J, Coste J, Shojaei T, et al. Risk factors for ectopic pregnancy: a comprehensive analysis based on a large case-control, population-based study in France. Am J Epidemiol 2003; 157(3): 185-94. DOI: https://doi.org/10.1093/aje/kwf190
- Panelli DM, Phillips CH, Brady PC. Incidence, diagnosis and management of tubal and nontubal ectopic pregnancies: a review. Fertil Res Pract 2015; 1: 15. DOI: https://doi.org/10.1186/s40738-015-0008-z
- Chouinard M, Mayrand MH, Ayoub A, Healy-Profitós J, Auger N. Ectopic pregnancy and outcomes of future intrauterine pregnancy. Fertil Steril 2019; 112(1): 112-119. DOI: https://doi.org/10.1016/j.fertnstert.2019.03.019
- Bhattacharya S, McLernon DJ, Lee AJ, Bhattacharya S. Reproductive outcomes following ectopic pregnancy: register-based retrospective cohort study. PLoS Med 2012; 9(6): e1001243. DOI: https://doi.org/10.1371/journal.pmed.1001243
- Li C, Zhao WH, Zhu Q, et al. Risk factors for ectopic pregnancy: a multi-center case-control study. BMC Pregnancy Childbirth 2015; 15: 187. DOI: https://doi.org/10.1186/s12884-015-0613-1
- Assouni Mindjah YA, Essiben F, Foumane P, Dohbit JS, Mboudou ET. Risk factors for ectopic pregnancy in a population of Cameroonian women: A case-control study. PLoS One 2018; 13(12): e0207699. DOI: https://doi.org/10.1371/journal.pone.0207699
- Bronson R. Ectopic pregnancy - still a challenge. Fertil Steril 2018; 110(7): 1265-1266. DOI: https://doi.org/10.1016/j.fertnstert.2018.09.008
- Gaskins AJ, Missmer SA, Rich-Edwards JW, Williams PL, Souter I, Chavarro JE. Demographic, lifestyle, and reproductive risk factors for ectopic pregnancy. Fertil Steril 2018; 110(7): 1328-1337. DOI: https://doi.org/10.1016/j.fertnstert.2018.08.022
- Asah-Opoku K, Oppong SA, Ameme DK, et al. Risk factors for ectopic pregnancy among pregnant women attending a tertiary healthcare facility in Accra, Ghana. Int J Gynaecol Obstet 2019; doi: 10.1002/ijgo.12928. DOI: https://doi.org/10.1002/ijgo.12928
- Kumar V, Gupta J. Tubal ectopic pregnancy. BMJ Clin Evid 2015; 11: 1406. PMID: https://www.ncbi.nlm.nih.gov/pubmed/26571203
- Job-Spira N, Fernandez H, Bouyer J, Pouly JL, Germain E, Coste J. Ruptured tubal ectopic pregnancy: risk factors and reproductive outcome. Results of a population-based study in France. Am J Obstet Gynecol 1999; 180: 938–44. DOI: https://doi.org/10.1016/s0002-9378(99)70665-4
- Bickell NA, Bodian C, Anderson RM, Kase N. Time and the risk of ruptured tubal pregnancy. Obstet Gynecol 2004; 104(4): 789-94. DOI: https://doi.org/10.1097/01.AOG.0000139912.65553.58
- Singh KB, Poole CA, Otterson WN, Dunnihoo DR, Bairnsfather LE, Long DC. Characteristics of indigent women with ruptured and unruptured tubal pregnancies. J Reprod Med 1992; 37: 745–8. PMID: https://www.ncbi.nlm.nih.gov/pubmed/1432993
- Ashfaq S, Sultan S, Aziz S, Irfan MM, Hasan M, Siddique A. Ectopic Pregnancy With Tubal Rupture: An Analysis Of 80 Cases. J Ayub Med Coll Abbottabad 2017; 29(2): 254-257. PMID: https://www.ncbi.nlm.nih.gov/pubmed/28718242
- Stulberg DB, Cain L, Dahlquist IH, Lauderdale DS. Ectopic pregnancy morbidity and mortality in low-income women, 2004-2008. Hum Reprod 2016; 31(3): 666-71. DOI: https://doi.org/10.1093/humrep/dev332
- Creanga AA, Shapiro-Mendoza CK, Bish CL, Zane S, Berg CJ, Callaghan WM. Trends in ectopic pregnancy mortality in the United States: 1980-2007. Obstet Gynecol 2011; 117(4): 837-43. DOI: https://doi.org/10.1097/AOG.0b013e3182113c10
- Goyaux N, Leke R, Keita N, Thonneau P. Ectopic pregnancy in African developing countries. Acta Obstet Gynecol Scand 2003; 82(4): 305-12. DOI: https://doi.org/10.1034/j.1600-0412.2003.00175.x
GMatH (Global Maternal Health) Model - Last updated: 28 November 2022
© Copyright 2020-2022 Zachary J. Ward
zward@hsph.harvard.edu