Hypertension Management
Model Inputs \(\rightarrow\) Clinical Interventions \(\rightarrow\) Hypertension Management
Overview
A review of historical declines in PE/E mortality in high-income countries found that the the most important interventions were widespread use of prenatal care with blood pressure and urine protein measurement, and increased access to hospital care for timely induction of labor or cesarean delivery for women with severe PE/E.[1] While the only definitive cure for pre-eclampsia is delivery of the baby (by induction of labor or pre-labor caesarean section), the mainstays of treatment are antihypertensive drugs for blood pressure control and magnesium sulphate (MgSO4) for eclampsia.[2]
Data
The only interventions which have been shown to prevent pre-eclampsia are anti-platelet agents, primarily low-dose aspirin, and calcium supplementation. For example, a Cochrane review found an 18% reduction in the risk of pre-eclampsia associated with the use of antiplatelet agents.[3] Currently, professional bodies including the American College of Obstetricians and Gynecologists in the US and the National Institute for Health and Care Excellence in the UK suggest prophylactic use of aspirin for women considered at high risk for pre-eclampsia.[4] A systematic review and metaanalysis found that administration of aspirin was associated with a reduction in the risk of preterm pre-eclampsia (RR 0.62, 95% CI 0.45-0.87), but that there was no significant effect on term pre-eclampsia (RR 0.92, 95% CI 0.70-1.21).[5] A Cochrane review found that calcium supplementation with at least 1 g of calcium could reduce high blood pressure (RR 0.65, 95% CI 0.53-0.81) as well as the risk of pre-eclampsia (RR 0.45, 95% CI 0.31-0.65) amongst pregnant women, with the greatest risk reduction occurring for women at high risk of pre-eclampsia.[6] To reduce the risk of pre-eclampsia, the WHO therefore recommends daily calcium supplementation (1.5-2.0 g oral elemental calcium) for populations with low dietary calcium intake.[7] However, except for calcium supplementation for women with low dietary calcium intake, all other preventive interventions need further assessment and are not recommended for use outside the context of clinical trials.[8] We therefore do not model specific interventions to prevent pre-eclampsia/eclampsia in the model, but do model the effect of management that can prevent pre-eclampsia from progressing to eclampsia.
While data from trials are insufficiently conclusive as to the optimal timing of delivery with pre-eclampsia, there is robust evidence that magnesium sulfate can prevent and control eclamptic seizures, and for pre-eclampsia, reduces the risk of eclampsia by more than 50%.[9,10,11] A Cochrane Review of magnesium sulphate and other anticonvulsants for women with pre-eclampsia compiled evidence from 6 trials, the largest of which was the Magpie Trial Collaborative Group.[12] This review found a 59% reduction in risk of eclampsia in women with pre-eclampsia (RR 0.41, 95% CI 0.29-0.58) and a 46% reduction (RR 0.54, 95% CI 0.26-1.10) in the risk of dying in women with pre-eclampsia randomized to magnesium sulfate.[12] A review showed that magnesium sulphate was the better anticonvulsant choice when treating women with eclampsia, and substantially reduced the risk of further seizures when compared to diazepam.[9] A second, smaller, review showed that magnesium sulphate performed better than a lytic cocktail at preventing maternal deaths as well as preventing breathing problems and convulsions.[12] One goal of this study was whether induction of labor in women with pregnancy induced hypertension or pre-eclampsia at term reduced costs and improved quality of life as compared to expectant monitoring. Two studies looked only at mild pre-eclampsia and gestational diabetes, but not at cases of maternal or neonatal death or eclampsia.[13,14] A study by one collaborative group found that the use of magnesium sulphate for women with pre-eclampsia was associated with a 16% reduction in the risk of death or serious morbidity related to pre-eclampsia 2 to 3 years later.[15]
Repeated doses of nifedipine, intravenous hydralazine, or labetalol every 15–30 min have been found to achieve blood pressure control in at least 80% of women - however, oral labetalol is not widely available in low-income and middle-income countries.[8] The availability of magnesium sulphate also varies by location. For example, one assessment of facilities in Karnataka found that magnesium sulphate was available in 18% of primary health centers, 48% of higher public facilities, and 70% of private facilities.[16] A follow up study in Karnataka found that in public facilities, magnesium sulphate was available in 60% of primary health centres, 68% of community health centers, and 100% of sub-district and district hospitals, while 92% of private facilities reported availability of magnesium sulphate.[17]
A Cochrane review of planned c-section versus planned vaginal birth for severe pre-eclampsia did not find any randomized clinical trials, and concludes that there are no rigorous data to assess whether c-section or vaginal birth is better for pregnant women with severe pre-eclampsia, and their babies.[18] However, reported rates of c-section for women with eclampsia are high. For example, a study in Mali of 116 women with eclampsia reported that cesarean section was performed in 77.6% of cases, and magnesium sulphate was used in 75% of cases.[19] A study in French Guiana in 2019 found that of 156 deliveries diagnosed with a hypertensive disorder, delivery was by cesarean in 49.7% of cases.[20] Another study in Zambia reported that c-section was performed for 7/8 cases of eclampsia.[21] However, while commonly used for women with eclampsia, c-sections resulting from eclampsia comprise a small percentage of all c-sections - a multi-country study in sub-Saharan Africa found that PE/E accounted for only 2.4% of all c-sections.[22] Given the uncertainty around the relative efficacy of c-section vs other methods of induced labor, we do not model differential mortality/morbidity by use of c-section, but include a probability of c-section for women with eclampsia to better match reported estimates of total c-sections, and to account for the higher risks of sepsis associated with emergency c-section.
Parameters
For women with PE/E, we model the availability and efficacy of PE/E management, conditional on delivery site. We assume that the availability of treatment increases by site and income group, and that treatment is not available at home. We assume that treatment availability increases over time and so constrain the year coefficient to be non-negative when sampling. We also assume that a proportion of women with eclampsia at an EmOC facility will undergo c-section. For simplicity, we assume that the efficacy of treatment is non-differential by PE/E severity, and apply the same risk reduction for all mortality and morbidity outcomes. However, we assume that severe PE/E can only be treated at CEmOC facilities.
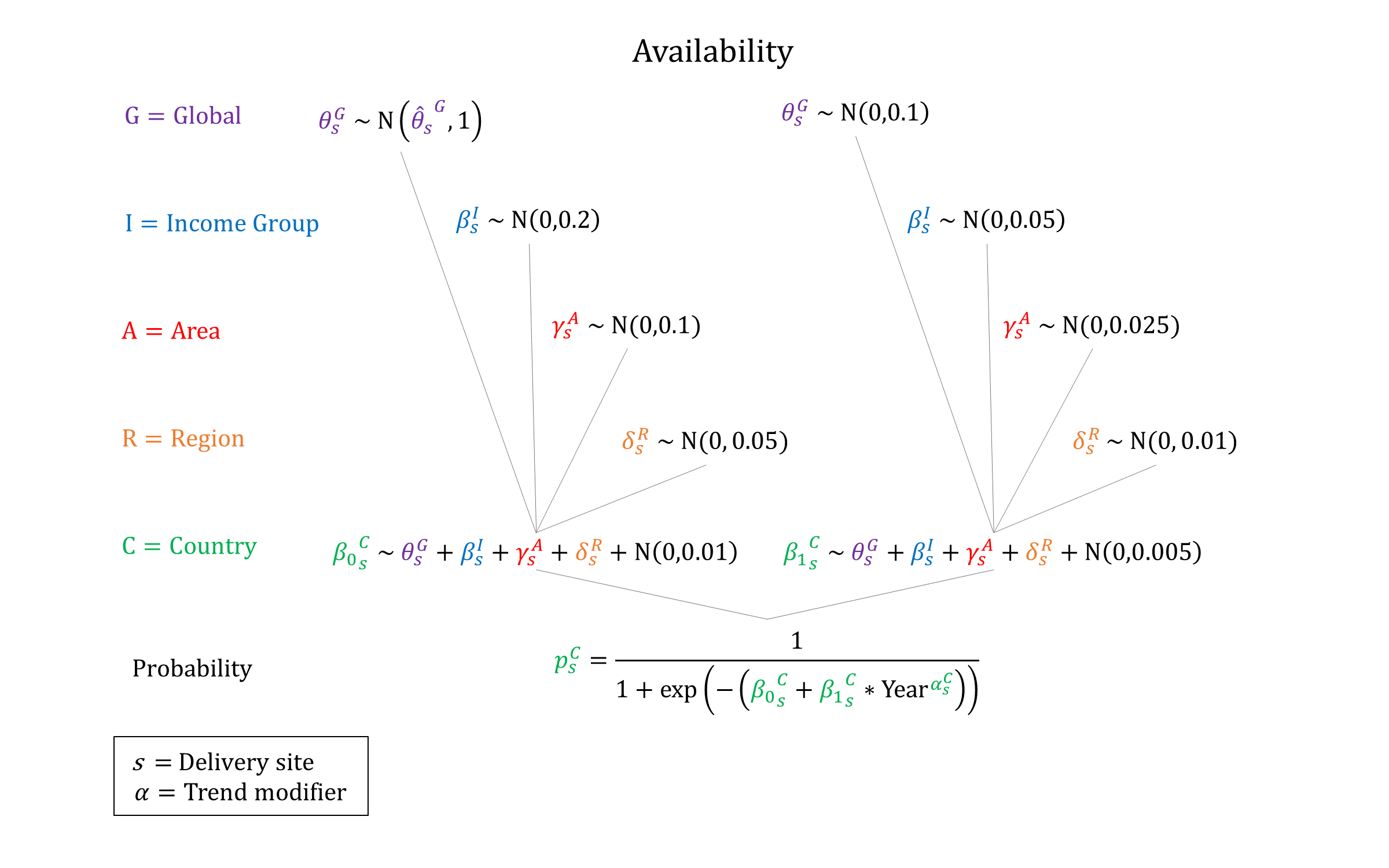
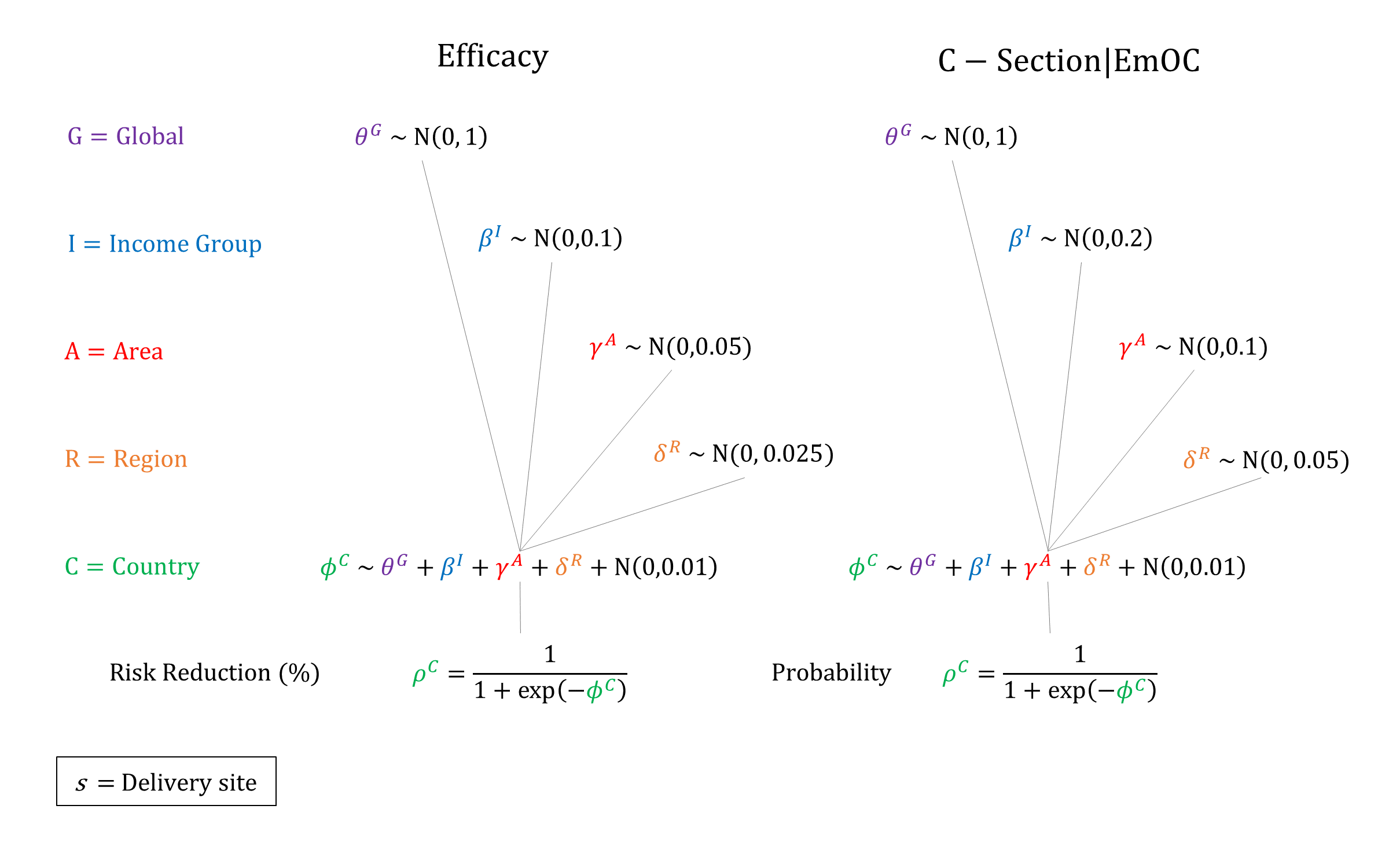
Priors
Model Implementation
Conditional on the delivery site we simulate the probabilities that treatment will be available and the efficacy of treatment on PE/E outcomes, accounting for quality of care.
References
- Goldenberg RL, McClure EM, Macguire ER, Kamath BD, Jobe AH. Lessons for low‐income regions following the reduction in hypertension‐related maternal mortality in high‐income countries. Int J Gynaecol Obstet 2011; 113: 91-5. DOI: https://doi.org/10.1016/j.ijgo.2011.01.002
- Gülmezoglu, Lawrie TA, Hezelgrave N, et al. Chapter 7. Interventions to Reduce Maternal and Child Morbidity and Mortality. In: Black RE, Laxminarayan L, Walker N, eds. Reproductive, Maternal, Newborn, and Child Health: Disease Control Priorities, Third Edition (Volume 2). Washington (DC): The International Bank for Reconstruction and Development / The World Bank; 2016. DOI: https://doi.org/10.1596/978-1-4648-0348-2_ch7
- Duley L, Meher S, Hunter KE, Seidler A, Askie LM. Antiplatelet agents for preventing pre-eclampsia and its complications. Cochrane Database Syst Rev 2019; 10: CD004659. DOI: https://doi.org/10.1002/14651858.cd004659.pub3
- Voutetakis A, Pervanidou P, Kanaka-Gantenbein C. Aspirin for the Prevention of Preeclampsia and Potential Consequences for Fetal Brain Development. JAMA Pediatr 2019; 173(7): 619-620. DOI: https://doi.org/10.1001/jamapediatrics.2019.1260
- Roberge S, Bujold E, Nicolaides KH. Aspirin for the prevention of preterm and term preeclampsia: systematic review and metaanalysis. Am J Obstet Gynecol 2018; 218(3): 287-293.e1. DOI: https://doi.org/10.1016/j.ajog.2017.11.561
- Hofmeyr GJ, Lawrie TA, Atallah ÁN, Torloni MR. Calcium supplementation during pregnancy for preventing hypertensive disorders and related problems. Cochrane Database Syst Rev 2018; 10: CD001059. DOI: https://doi.org/10.1002/14651858.cd001059.pub5
- World Health Organization. WHO recommendations on antenatal care for a positive pregnancy experience. 2016. Available at: http://apps.who.int/iris/bitstream/10665/250796/1/9789241549912-eng.pdf
- Mol BWJ, Roberts CT, Thangaratinam S, Magee LA, de Groot CJM, Hofmeyr GJ. Pre-eclampsia. Lancet 2016; 387(10022): 999-1011. DOI: https://doi.org/10.1016/s0140-6736(15)00070-7
- Duley L. The global impact of pre-eclampsia and eclampsia. Semin Perinatol 2009; 33(3): 130-7. DOI: https://doi.org/10.1053/j.semperi.2009.02.010
- Langer A, Villar J, Tell K, Kim T, Kennedy S. Reducing eclampsia-related deaths–a call to action. Lancet 2008; 371(9614): 705-6. DOI: https://doi.org/10.1016/s0140-6736(08)60321-9
- Tukur J. The use of magnesium sulphate for the treatment of severe pre-eclampsia and eclampsia. Ann Afr Med 2009; 8(2): 76-80. DOI: https://doi.org/10.4103/1596-3519.56232
- Duley L, Gülmezoglu AM, Henderson-Smart DJ, Chou D. Magnesium sulphate and other anticonvulsants for women with pre-eclampsia. Cochrane Database Syst Rev 2010; 11: CD000025. DOI: https://doi.org/10.1002/14651858.cd000025.pub2
- Koopmans CM, Bijlenga D, Aarnoudse JG, et al. Induction of labour versus expectant monitoring in women with pregnancy induced hypertension or mild preeclampsia at term: the HYPITAT trial. BMC Pregnancy Childbirth 2007; 7: 14. DOI: https://doi.org/10.1186/1471-2393-7-14
- Koopmans CM, Bijlenga D, Groen H, et al. Induction of labour versus expectant monitoring for gestational hypertension or mild pre-eclampsia after 36 weeks’ gestation (HYPITAT): a multicentre, open-label randomised controlled trial. Lancet 2009; 374(9694): 979-88. DOI: https://doi.org/10.1016/s0140-6736(09)60736-4
- Magpie Trial Follow-Up Study Collaborative Group. The Magpie Trial: a randomised trial comparing magnesium sulphate with placebo for pre-eclampsia. Outcome for women at 2 years. BJOG 2007; 114(3): 300-9. DOI: https://doi.org/10.1111/j.1471-0528.2006.01166.x
- Jayanna K, Mony P, Ramesh BM, et al. Assessment of facility readiness and provider preparedness for dealing with postpartum haemorrhage and pre-eclampsia/eclampsia in public and private health facilities of northern Karnataka, India: a cross-sectional study. BMC Pregnancy Childbirth 2014; 14: 304. DOI: https://doi.org/10.1186/1471-2393-14-304
- Katageri G, Charantimath U, Joshi A, et al. Availability and use of magnesium sulphate at health care facilities in two selected districts of North Karnataka, India. Reprod Health 2018; 15(Suppl 1): 91. DOI: https://doi.org/10.1186/s12978-018-0531-6
- Amorim MMR, Souza ASR, Katz L. Planned caesarean section versus planned vaginal birth for severe pre‐eclampsia. Cochrane Database Syst Rev 2017; 10: CD009430. DOI: https://dx.doi.org/10.1002%2F14651858.CD009430.pub2
- Kampo MI, Sogoba S, Kassogué D. [Maternal and perinatal prognosis of eclampsia at the Timbuktu Hospital in Mali]. Pan Afr Med J 2020; 36: 175. DOI: https://doi.org/10.11604/pamj.2020.36.175.17976
- Mhiri R, Mvogo A, Kamga A, et al. Epidemiology and maternal prognosis of hypertension disorders of pregnancy in French Guiana. Pregnancy Hypertens 2020; 20: 96-101. DOI: https://doi.org/10.1016/j.preghy.2020.03.010
- Miyoshi Y, Matsubara K, Takata N, Oka Y. Significance of pre-hospital care to reduce the morbidity of eclampsia in rural Zambia. Pregnancy Hypertens 2019; 17: 100-103. DOI: https://doi.org/10.1016/j.preghy.2019.05.008
- Chu K, Cortier H, Maldonado F, Mashant T, Ford N, Trelles M. Cesarean section rates and indications in sub-Saharan Africa: a multi-country study from Medecins sans Frontieres. PLoS One 2012; 7(9): e44484. DOI: https://doi.org/10.1371/journal.pone.0044484
GMatH (Global Maternal Health) Model - Last updated: 28 November 2022
© Copyright 2020-2022 Zachary J. Ward
zward@hsph.harvard.edu