Management of Ectopic Pregnancy
Model Inputs \(\rightarrow\) Clinical Interventions \(\rightarrow\) Management of Ectopic Pregnancy
Overview
Ectopic pregnancy is a difficult diagnosis and may be missed initially. While diagnosis is often delayed due to very early presentations, it can also be missed because patients may not have the same risk factors or symptoms, such as serum B-human chorionic gonadotropin levels/trends or ultrasound findings.[1] Most cases of tubal ectopic pregnancy that are detected early can be successfully treated with methotrexate alone or minimally invasive surgery.[2] However, tubal ectopic pregnancy in an unstable patient requires prompt surgical intervention,[2] and is indicated in patients with hemodynamic compromise or other clinical signs of ruptured ectopic pregnancy including pain or evidence of intra-abdominal bleeding.[3] Laparoscopic surgery has been found to be a cost-effective treatment in the surgical management of tubal ectopic pregnancy.[4]
Data
Ectopic pregnancy can be associated with severe maternal morbidity, with up to a third of women being managed after the pregnancy has ruptured.[5] While 15% to 40% of ectopic pregnancies may be suitable for non-surgical management (expected management or methotrexate treatment),[6] despite initial medical treatment, around 15% of women eventually require surgery.[7]
A study of 221 women with tubal pregnancy in the US found that physicians were certain of the diagnosis at the first examination for only 48% of women.[8] Overall, 32% of women experienced tubal rupture (n = 71), and 12 women with rupture and 3 without received a blood transfusion. Ten women were treated with methotrexate only, 21 received methotrexate and subsequently underwent surgical treatment, 51 underwent salpingostomy, and 160 had salpingectomy.[8] Another study in northeast Brazil found that 63.4% of patients presented with ruptured ectopic pregnancy and 5.9% had hemodynamic instability.[9] Among the patients with ruptured ectopic pregnancy, 61% had already sought care at another center. With regard to initial treatment, 78.2% underwent surgery (27.2%, laparoscopy), 16.8% used methotrexate, and 5% underwent expectant management. However, among those who received methotrexate, 41.2% needed subsequent surgery because of elevated blood-hCG level (57.1%) and clinical signs of ruptured ectopic pregnancy (42.9%).[9]
Although complicated ectopic pregnancy was nearly universally fatal until the beginning of the nineteenth century, earlier diagnosis and improvements in technology have resulted in substantially decreased mortality in high income countries, with current mortality rates estimated at 0.35/1000 in the UK and 0.5/1000 in the USA.[5]
Parameters
We model the availability and efficacy of ectopic pregnancy treatment. Because surgery is often needed, we assume that the availability of treatment increases by site and income group, and that treatment is not available at home. We assume that the availability of treatment increases over time and so constrain the year coefficient to be non-negative when sampling. Given the low mortality rates from ectopic pregnancy in high income countries, we assume that the efficacy of treatment is very high and set priors around 99%.
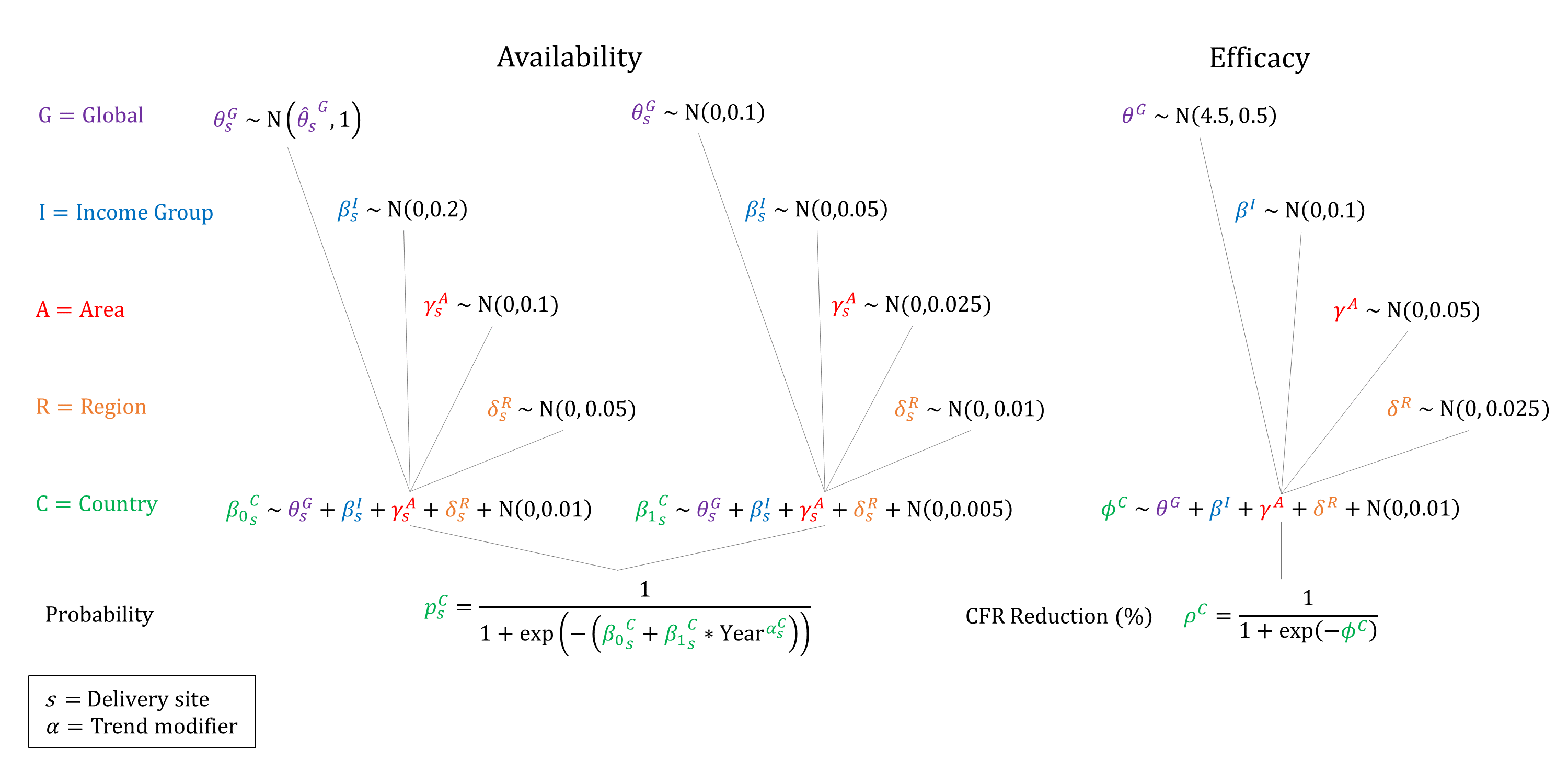
Priors
Model Implementation
We assume that women are recognized/referred for ruptured ectopic pregnancy from home. Conditional on the site of treatment we simulate the probabilities that treatment will be available and the efficacy of treatment on ectopic pregnancy mortality.
References
- Robertson JJ, Long B, Koyfman A. Emergency Medicine Myths: Ectopic Pregnancy Evaluation, Risk Factors, and Presentation. J Emerg Med 2017; 53(6): 819-828. DOI: https://doi.org/10.1016/j.jemermed.2017.08.074
- ACOG Practice Bulletin No. 193 Summary: Tubal Ectopic Pregnancy. Obstet Gynecol 2018; 131(3): 613-615. DOI: https://doi.org/10.1097/aog.0000000000002559
- Panelli DM, Phillips CH, Brady PC. Incidence, diagnosis and management of tubal and nontubal ectopic pregnancies: a review. Fertil Res Pract 2015; 1: 15. DOI: https://doi.org/10.1186/s40738-015-0008-z
- Hajenius PJ, Mol F, Mol BWJ, Bossuyt PMM, Ankum WM, van der Veen F. Interventions for tubal ectopic pregnancy. Cochrane Database Syst Rev 2007; 1: CD000324. DOI: https://doi.org/10.1002/14651858.cd000324.pub2
- McGurk L, Oliver R, Odejinmi F. Severe morbidity with ectopic pregnancy is associated with late presentation. J Obstet Gynaecol 2019; 39(5): 670-674. DOI: https://doi.org/10.1080/01443615.2018.1557610
- Kumar V, Gupta J. Tubal ectopic pregnancy. BMJ Clin Evid 2015; 11: 1406. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4646159/
- Lee JH, Kim S, Lee I, et al. A risk prediction model for medical treatment failure in tubal pregnancy. Eur J Obstet Gynecol Reprod Biol 2018; 225: 148-154. DOI: https://doi.org/10.1016/j.ejogrb.2018.04.020
- Bickell NA, Bodian C, Anderson RM, Kase N. Time and the risk of ruptured tubal pregnancy. Obstet Gynecol 2004; 104(4): 789-94. DOI: https://doi.org/10.1097/01.aog.0000139912.65553.58
- Cordeiro FDE, Alves GJA, Araujo Júnior E, Feitosa LEE. Ectopic pregnancies: a retrospective cohort analysis in a tertiary reference center in the Northeast Region of Brazil. Ceska Gynekol 2018; 83(6): 434-439. PMID: https://pubmed.ncbi.nlm.nih.gov/30848148/
GMatH (Global Maternal Health) Model - Last updated: 28 November 2022
© Copyright 2020-2022 Zachary J. Ward
zward@hsph.harvard.edu